[ad_1]
Pfizer has begun testing its COVID-19 vaccine on teens and preteens — amid concerns among some US pediatricians that they might not know if any shots can protect children from the virus in time for the next school year.
The pharmaceutical giant last week received permission to test its vaccine on US kids as young as 12 — one of only a handful of global attempts to determine whether any shots being tested on adults can also be effective in children.
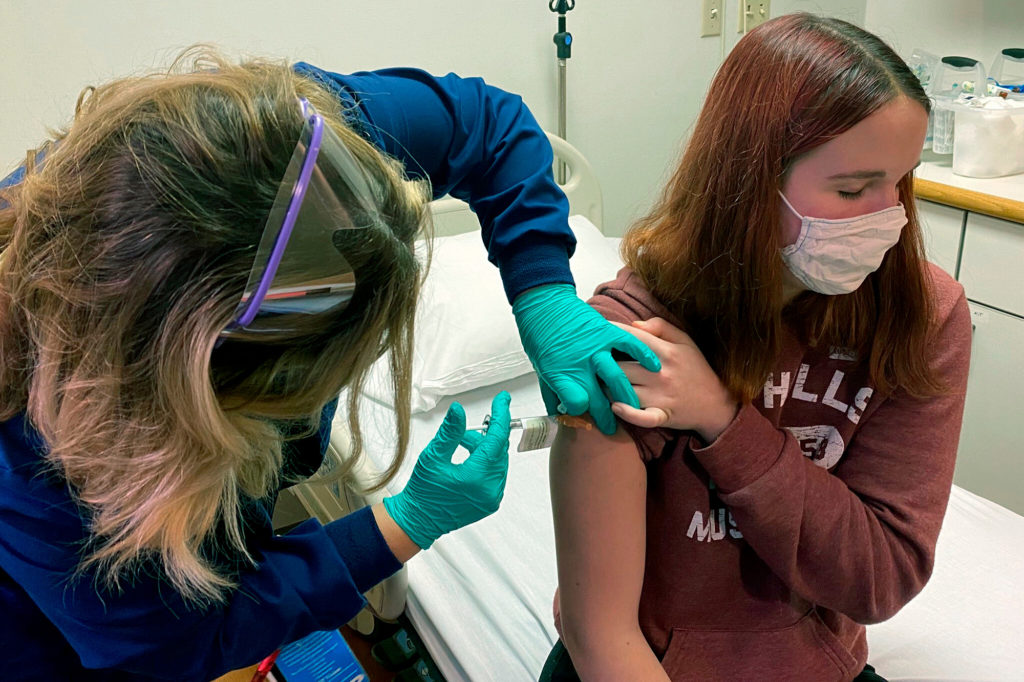
Katelyn Evans, 16, was the first teen to get an injection in the Pfizer study at Cincinnati Children’s Hospital.
“I just figured the more people they have to do tests on, the quicker they can put out a vaccine and people can be safe and healthy,” Evans said.
Multiple vaccine candidates are in final-stage studies in tens of thousands of adults, but it’s unlikely they’ll be recommended for children.
Vaccines can’t be administered to children unless they’ve been tested in their age group.
“The public doesn’t understand that,” Dr. Evan Anderson of Emory University, who has been pushing for pediatric testing of COVID-19 vaccines, told the Associated Press.
Clinical research coordinator Tammy Lewis-McCauley administers an injection to Katelyn Evan as part of Pfizer’s COVID-19 vaccine trial at Cincinnati Children’s Hospital Medical Center last week.Cincinnati Children’s Hospital Medical Center via AP
He said he’s encouraged by Pfizer’s study in adolescents, but finds it “very concerning” that children younger than 12 may not have a vaccine by next fall.
Children represent about 10 percent of confirmed COVID-19 cases in the US — and while they are far less likely than adults to experience severe symptoms, about 120 have died of the virus nationwide, according to a tally by the American Academy of Pediatrics.
About the same number of US children die from the flu in an average year.
A small number of children have additionally developed a serious inflammatory condition linked to the coronavirus.
And in general, COVID-19 has a greater impact on kids than some other diseases that require routine pediatric vaccinations, according to Anderson.
Besides the Pfizer study, Moderna Inc., Johnson & Johnson and Novavax all hope to begin some pediatric research later this year, for various age groups.
In China, Sinovac and SinoPharm have opened studies in which children as young as 3 can participate.
A British study of a vaccine by AstraZeneca allows a low dose to be tested in certain children, but the company says it won’t be recruiting youngsters until “sufficient” safety data is compiled for adults.
With Post wires
[ad_2]
Source link